Therapeutic Plasma Exchange
First-line treatment for multiple diseases
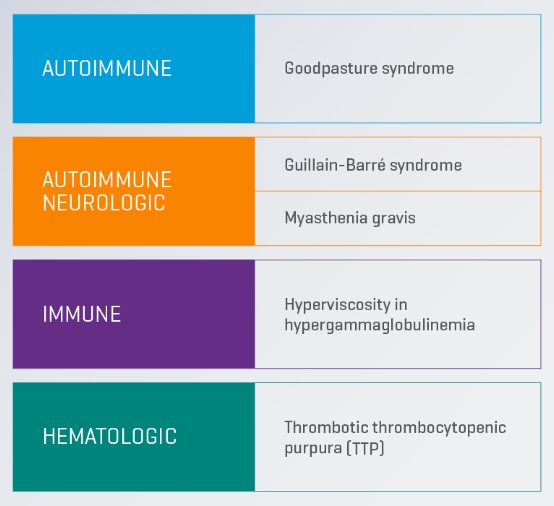
Therapeutic Plasma Exchange (TPE) is associated with positive improvement in clinical outcomes in the treatment of Guillain-Barré syndrome, Thrombotic Thrombocytopenic Purpura (TTP), Myasthenia Gravis and Goodpasture syndrome and is recommended as a first-line therapy* for these conditions in guidelines from the American Society for Apheresis (ASFA).2
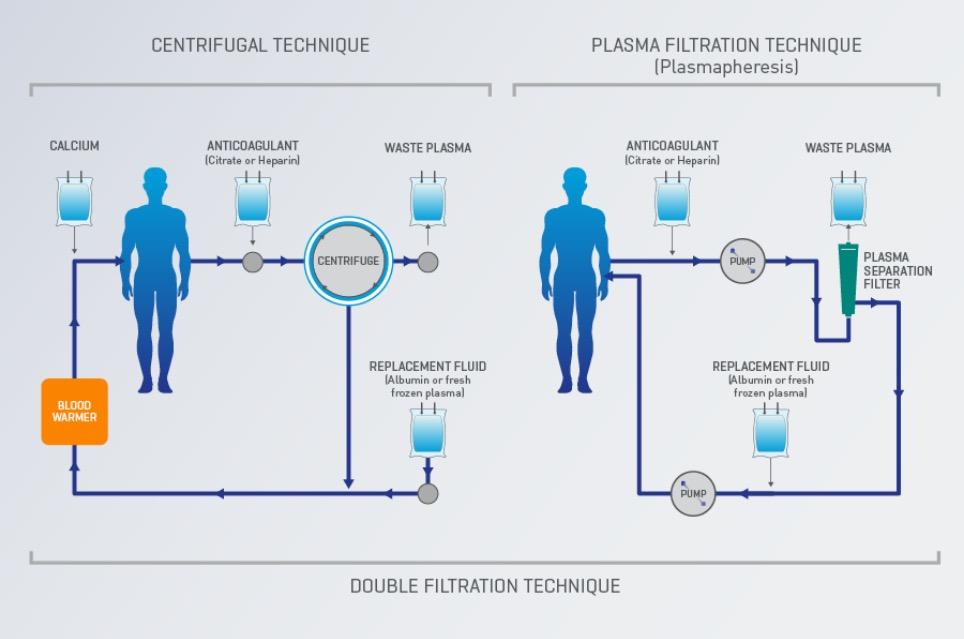
The goal of Therapeutic Plasma Exchange is to remove and/or treat diseased blood components.
* Recommended as first line therapy, either as the primary standalone treatment or in conjunction with other modes of treatment for certain indications of Goodpasture syndrome, Myasthenia gravis, and other diseases noted above.
How Therapeutic Plasma Exchange works
During apheresis therapy, a component of the blood that is abnormal and causing illness is removed while the healthy, normal components are returned to the patient.
Therapeutic Plasma Exchange is a type of apheresis therapy where plasma is removed through the large pore membrane of the plasma filter, while fresh plasma or other types of colloid solutions are infused post plasma filter to replace the plasma removed. Therapeutic Plasma Exchange is designed for the removal of large-molecular weight substances such as pathogenic auto-antibodies, immune complexes, cryoglobulins, and more.1
Treatment indications for Therapeutic Plasma Exchange therapy have increased
Treatment indications for therapeutic plasma exchange have increased over time as shown in this graphic.
Therapeutic Plasma Exchange is associated with positive improvement in clinical outcomes
Guillain–Barré Syndrome
Several controlled trials indicate that TPE can accelerate motor recovery, decrease time on the ventilator and speed of attainment of other clinical milestones compared with supportive care alone.2
Thrombotic Thrombocytopenic Purpura (TTP)
TPE has decreased overall mortality of immune mediated TTP from nearly uniformly fatal to <10-20%.2
Myasthenia Gravis (MG)
TPE in MG has a rapid clinical effect but it may take a week; concomitant immunosuppression must be initiated or modified for sustained control of MG activity.2
Goodpasture Syndrome
A single randomized controlled trial involving a small number of patients showed that TPE maintained kidney function and improved survival.2
ASFA Guidelines
Comprehensive guidelines for therapeutic apheresis are published by the American Society for Apheresis (AFSA). The AFSA evidence-based approach assigns diseases to categories based on a stringent review of the literature, analysis of quality of evidence, and strength of recommendation derived from the evidence.